What is ORGOVYX?
Hear from real ORGOVYX patients
Watch real patients share their journeys with advanced prostate cancer and how ORGOVYX fits into their treatment plan.
The lowdown on how ORGOVYX works
Lowering testosterone is one of the main ways to treat advanced prostate cancer. And that's exactly what ORGOVYX does.
Imagine the body makes testosterone the way that water comes out of a faucet
Without ORGOVYX, the faucet is turned on and testosterone flows freely.
With ORGOVYX, the faucet is almost turned off and the flow of testosterone is slowed, meaning there's less testosterone.
ORGOVYX was studied in a clinical trial
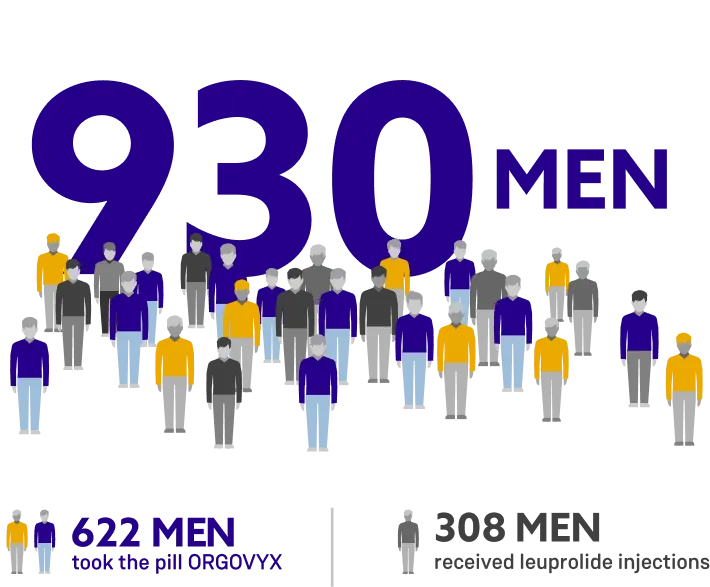
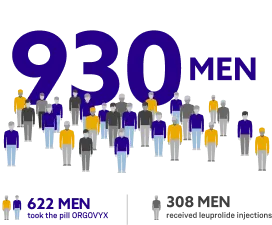
ORGOVYX was studied in a clinical trial in more than 900 men with advanced prostate cancer.
Men in the trial were treated for 48 weeks and either took the pill ORGOVYX or received leuprolide acetate injections.
The purpose of the trial was to measure how well ORGOVYX lowered testosterone to the treatment goal of below 50 ng/dL (nanograms per deciliter) from day 29 through week 48.
While it wasn't the main focus of the trial, the trial also monitored how much ORGOVYX was able to lower prostate-specific antigen (PSA) levels.‡
‡Because the clinical trial included many different types of patients, the results of PSA monitoring should be interpreted with caution. No evidence has shown that the speed of PSA decline is related to a clinical benefit.
SELECT IMPORTANT SAFETY INFORMATION
Most common side effects of ORGOVYX include:- hot flushes
- increased blood sugar levels
- increased blood fat (triglyceride) levels
- muscle and joint pain
- decreased blood hemoglobin levels
- increased liver enzymes
- tiredness
- constipation
- diarrhea
ORGOVYX may cause other side effects including weight gain, decreased sex drive, and erectile function problems.
ORGOVYX may cause fertility problems in males, which may affect your ability to father children. Talk to your healthcare provider if this is a concern for you.
These are not all the possible side effects of ORGOVYX. Call your doctor for medical advice about side effects or if you have a side effect that bothers you or does not go away.
You may report side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
Please see below for additional safety information.
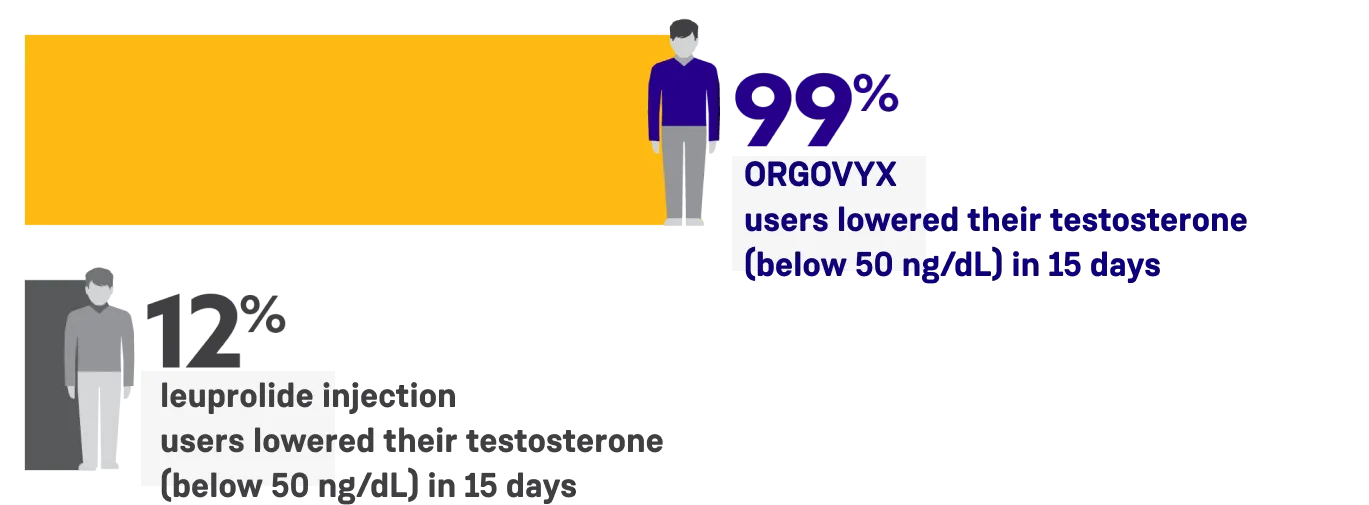
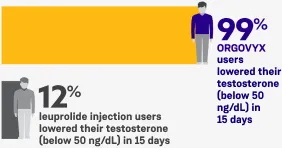
ORGOVYX was proven to lower testosterone levels fast
After 15 days of treatment, 99% of men taking ORGOVYX lowered their testosterone levels to below 50 ng/dL in the clinical trial and 12% of men receiving leuprolide injections lowered their testosterone levels to below 50 ng/dL.
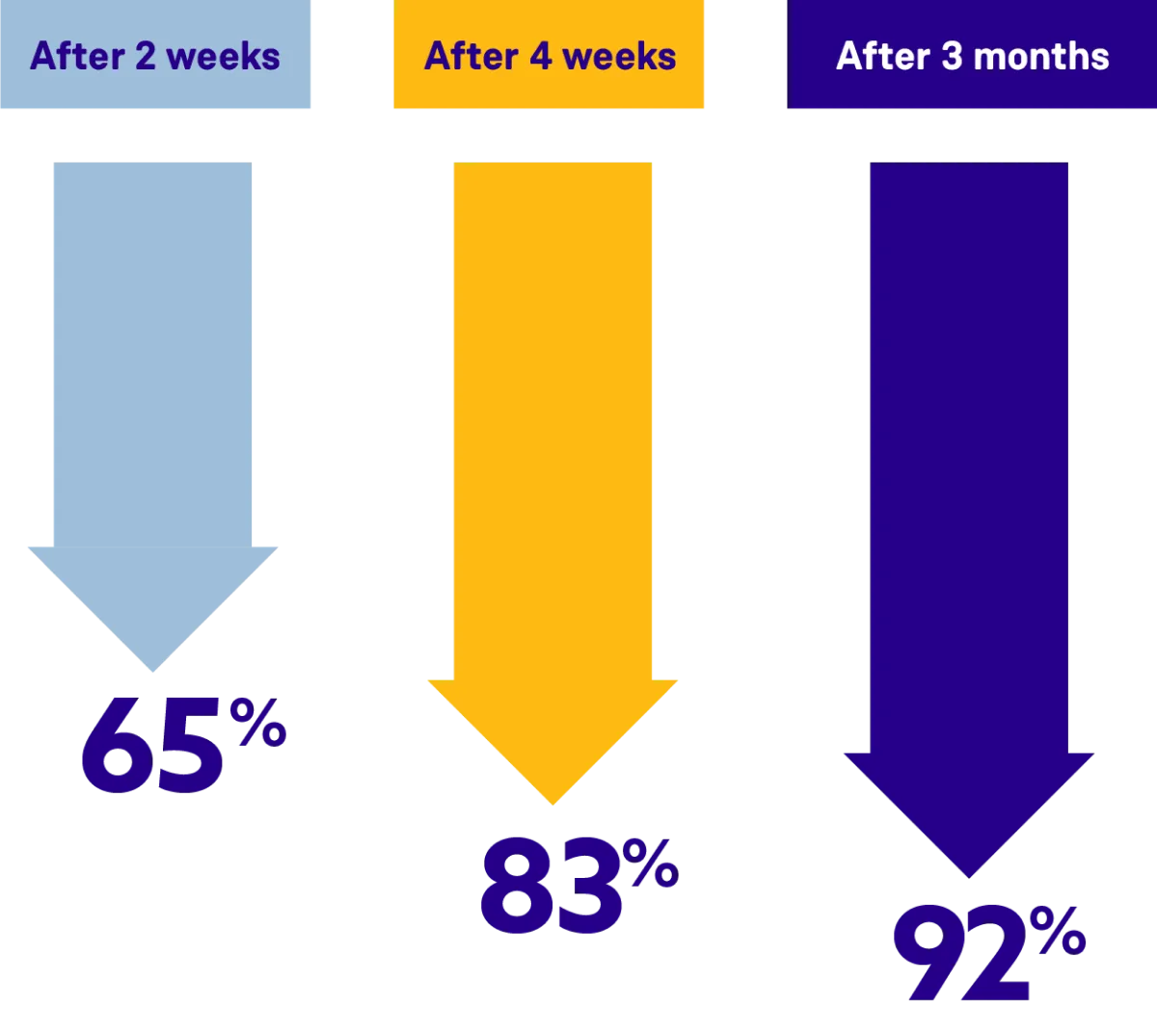
ORGOVYX lowered PSA levels, on average, by 92% after 3 months
In the clinical trial, PSA levels were monitored against the pretreatment level (baseline). During the first few months of taking ORGOVYX, men saw their PSA levels were lowered the longer they stayed on treatment, and kept them low throughout the 48-week trial.§
In the clinical trial, PSA levels were monitored against the pretreatment level (baseline). During the first few months of taking ORGOVYX, men saw their PSA levels were lowered the longer they stayed on treatment, and kept them low throughout the 48-week trial.§
It's important to know that everyone is unique and may see different results. Talk to your doctor about what these clinical trial results may mean for you.
§Even though it wasn't the main focus of the clinical trial, prostate-specific antigen (PSA) levels were monitored. Because the clinical trial included many different types of patients, the results of PSA monitoring should be interpreted with caution. No evidence has shown that the speed of PSA decline is related to a clinical benefit.
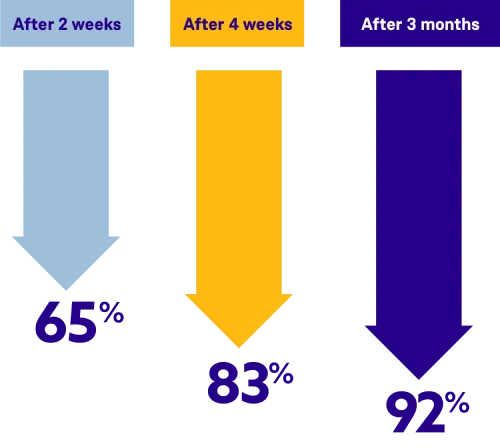
It's important to know that everyone is unique and may see different results. Talk to your doctor about what these clinical trial results may mean for you.
§Even though it wasn't the main focus of the clinical trial, PSA (prostate-specific antigen) levels were monitored. Because the clinical trial included many different types of patients, the results of PSA monitoring should be interpreted with caution. No evidence has shown that the speed of PSA decline is related to a clinical benefit.


Side effects
Before taking ORGOVYX, tell your doctor about all of your medical conditions, including:
- If you have any heart problems, including a condition called long QT syndrome
- If you are pregnant or plan to become pregnant. ORGOVYX can harm your unborn baby and cause loss of pregnancy (miscarriage)
- If you have a partner who is pregnant or may become pregnant. Men who have female partners who are able to become pregnant should use effective birth control (contraception) during treatment with ORGOVYX and for 2 weeks after the last dose of ORGOVYX
- If you are breastfeeding or plan to breastfeed. It is not known if ORGOVYX passes into your breast milk
What are the possible side effects?
ORGOVYX may cause serious side effects, such as changes in the electrical activity of your heart. This is called QT prolongation. Your doctor may check your salt levels, also known as electrolytes, and the electrical activity of your heart while you are taking ORGOVYX.
Tell your doctor right away if you have any of these signs or symptoms of QT prolongation:
- Dizziness
- Fainting
- Feeling that your heart is pounding or racing (palpitations)
- Chest pain
ORGOVYX may cause allergic reactions. Stop taking ORGOVYX and tell your healthcare provider or get emergency medical help right away if you get any signs or symptoms of an allergic reaction, including:
- Swelling of your face, lips, tongue, throat, or trouble swallowing
- Trouble breathing
- Hives (raised bumps), rash, or redness all over your body
Common side effects
The most common side effects men experienced while taking ORGOVYX were:
Hot flushes, also known as hot flashes
Increased blood sugar and/or increased blood fat (triglyceride) levels
Muscle and joint pain
Decreased blood hemoglobin levels
Constipation
Increased liver enzymes
Diarrhea
Tiredness
ORGOVYX may cause other side effects such as weight gain, decreased sex drive, and erectile function problems.
ORGOVYX may cause fertility problems, which may affect your ability to have children. Talk to your doctor if this is a concern for you.
These are not all the possible side effects of ORGOVYX. Call your doctor for medical advice about side effects or if you have a side effect that bothers you or does not go away.
You may report side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
Know what to ask your doctor?
We know there's a lot on your mind. There are so many questions to ask: How do I even begin? What do I say to my doctor when it comes to treating my advanced prostate cancer? It can be overwhelming. We have a resource that may help.